Christopher Koenigsmann

Associate Professor
General Chemistry, Materials Chemistry, Nanotechnology, and Renewable Energy
Email: [email protected]
Office: JMH 514
Lab: JMH 502
Phone: 718-817-4439
-
- Associate Professor at Fordham University
- Assistant Professor at Fordham University
- Postdoctoral Associate at Yale University, 2013 - 2015
- Ph.D. from Stony Brook University and Brookhaven National Laboratory, 2013
- B.S. from Fairfield University, 2008
-
I. Nanomaterials for Renewable Energy
There has been a recent and growing demand for green, renewable energy sources to replace fossil fuels. Our group focuses on two key areas in renewable energy:i.) Converting sunlight into electricity and fuels using photoelectrochemical cells: A promising approach to satisfying our nation’s energy needs is to convert solar energy either into electricity or directly into renewable fuels such as hydrogen. However, the efficiency of these energy conversion processes remains relatively low. In this context, we are focusing our efforts on synthesizing and tailoring the properties of nanostructured metal-containing materials to improve their photoelectrochemical performance in working devices. An important example includes the dye-sensitized photoelectrochemical cell (DSPC) device, which converts solar energy to either electricity or to solar fuels.
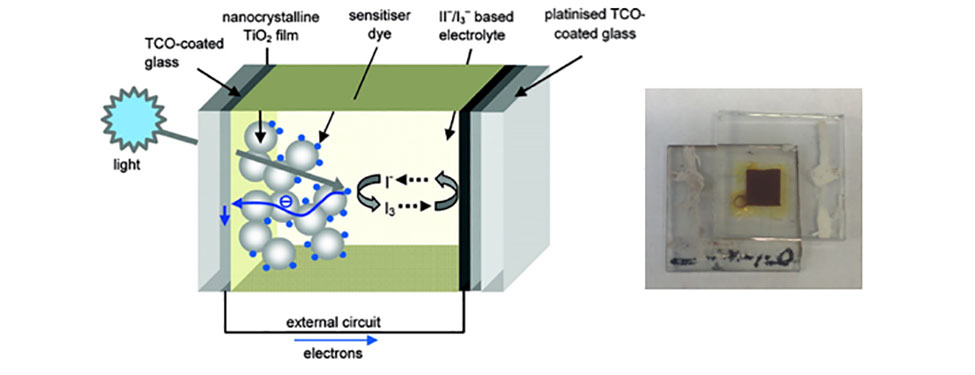
Converting Solar Energy to Electricity with DSPCs: Light from the sun is absorbed by a dye molecule leading to the excitation of electron from the ground state to the excited state. This excited electron is captured by the metal oxide nanoparticles that the dye is attached to and is passed through an external circuit producing electricity. The circuit is completed by an electrolyte with an iodine containing redox couple. These devices (right image) can be prepared in our laboratory using simple assembly techniques and have efficiencies of up to 9%. (Ref: Chemistry of Materials, 2011, 23, 3381-3399. Copyright 2011 American Chemical Society)ii.) Utilizing renewable fuels to power homes and automobiles with fuel cells: The electrochemical oxidation of the fuel and reduction of oxygen is facilitated by catalysts, which typically consist of small (2 – 4 nm) spherical platinum nanoparticles. Although platinum is the best element to catalyze the reactions, platinum’s extremely high cost and low abundance prevents large scale use of platinum in fuel cells. Our interest is in designing new fuel cell catalysts that have higher catalytic activity and better long-term durability, while utilizing abundant and less expensive metals. We accomplish this task by tuning the size, shape, and composition of the catalyst itself to improve overall performance.
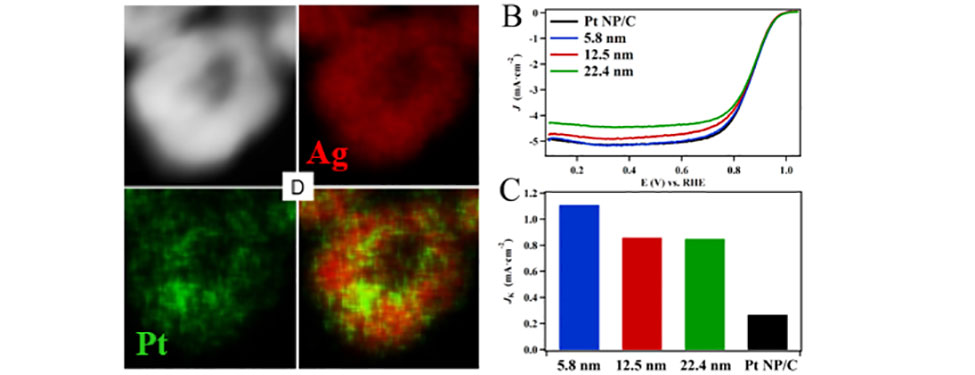
Hollow Nanoparticles in Action as Fuel Cell Catalysts: The image on the left depicts a high angle annular dark field (HAADF) TEM image of a 12.5 nm hollow Pt-Ag nanoparticle. The energy dispersive X-ray maps show that the Pt and Ag components are uniformly mixed in the particle’s shell. The hollow particples were found to have more than five-fold higher catalytic activity toward the oxygen reduction reaction than state-of-the-art commercial Pt nanoparticles. (Ref: RSC Advances 2017, 7, 46916-46924 – Reproduced by permission of The Royal Society of Chemistry)II. Electrochemical Sensors - Detecting Biologically Relevant Small Organic Molecules
The high cost of precious metals has also hindered their widespread use as electrocatalysts for the oxidation and detection of small organic molecules such as ethanol and glucose. Thus, the development of cost-effective electrocatalysts can have broad commercial implications and can contribute to broader global challenges in energy and health care costs. We are focusing our efforts on developing new nanostructured architectures as cost-effective catalysts with high sensitivity and selectivity for a wide range of small organic molecules.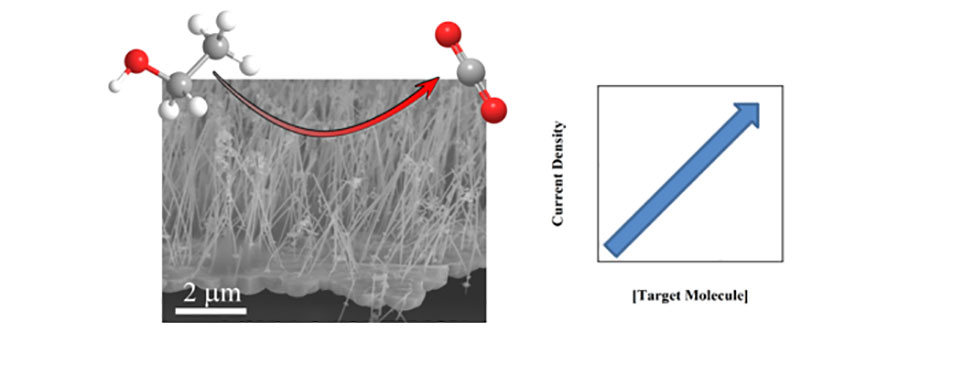
-
Please see Google Scholar for a complete list of publications.
McGuire, S.C.; Koenigsmann, C.; Chou, C.C.; Tong, X.; Wong, S.S., Lanthanum-Based Double Perovskite Nanoscale Motifs as Support Media for the Methanol Oxidation Reaction. Catalysis Science & Technology, 2022, 12 (2), 613-629.
Smina, N.; Rosen, A.; Sztaberek, L.; Beatrez, W.; Kingsbury, K.; Troia, R.; Wang, Y.; Zhao, J.; Schrier, J.;* Koenigsmann, C.,* Enhanced Electrocatalytic Oxidation of Small Organic Molecules on Platinum-Gold Nanowires: Influence of the Surface Structure and Pt-Pt/Pt-Au Pair Site Density. ACS Applied Materials & Interfaces 2021, 13 (50), 59892-59903.
Hurley, N.; Li, L.; Koenigsmann, C.; Wong, S.S., Surfactant-Free Synthesis of Three-Dimensional Perovskite Titania-Based Micron-Scale Motifs Used as Catalytic Supports for the Methanol Oxidation Reaction. Molecules 2021, 26 (4), 909.
Wang, Y.; Chen, S.; Wang, X.; Rosen, A.; Beatrez, W.; Sztaberek, L.; Haiyan, T.; Zhang, L.; Koenigsmann, C.*; Zhao, J., Composition-Dependent Oxygen Reduction Reaction Activity of Pt-Surfaced PtNi Dodecahedral Nanoframes. ACS Applied Energy Materials 2020, 3 (1), 786-776.
Sztaberek, L.; Mabey, H.; Beatrez, W.; Lore, C.; Santulli, A.C.; Koenigsmann, C., Sol-Gel Synthesis of Ruthenium Oxide Nanowires to Enhance Methanol Oxidation in Supported Platinum Nanoparticle Catalysts. ACS Omega 2019, 4, 10, 14226–14233. (DOI: https://doi.org/10.1021/acsomega.9b01489)
Banerjee, S.; Liu, C.H.; Lee, J.D.; Kovyakh, A.; Grasmik, V.; Prymak, O.; Koenigsmann, C.; Liu, H.; Wang, L.; Abeykoon, A.M.M.; Wong, S.S.; Epple, M.; Murray, C.B.; Billinge, S.J.L., Improved Models for Metallic Nanoparticle Cores from Atomic Pair Distribution Function (PDF) Analysis. The Journal of Physical Chemistry C. 2018, 122, 29498-29506. (DOI: 10.1021/acs.jpcc.8b05897)
Shutang, C.; Thota, S.; Singh, G.; Aímola, T. J.; Koenigsmann, C.; Zhao, J., Synthesis of Hollow Pt-Ag Nanoparticles by Oxygen-Assisted Acid Etching as Electrocatalysts for the Oxygen Reduction Reaction. RSC Advances 2017, 7, 46916 – 46924.
Colliard, I.; Koenigsmann, C., One-Dimensional Nanostructured Catalysts for the Oxygen Reduction Reaction. In One-Dimensional Nanostructures for PEM Fuel Cell Applications, 1st ed.; Pollet, B. G., Ed. Elsevier: London, United Kingdom, 2017; pp 19-48.