Nicholas Sawyer

Assistant Professor
Bioorganic Chemistry/Chemical Biology
Email: [email protected]
Office: JMH 638
Lab: JMH 633/640
Phone: 718-817-4461
-
- Assistant Professor at Fordham University
- NIH NRSA Postdoctoral Fellow at New York University
- Ph.D. in Molecular Biophysics and Biochemistry from Yale University
- B.Sc. in Biochemistry from Rutgers University
-
Research in my group aims to synthesize and evaluate molecules to modulate the function of protein-protein interactions involved in human health and disease. In particular, we focus on molecules that imitate interacting protein surfaces, especially peptides. Such molecules have utility as both probes for understanding essential biochemical processes and for innovation in drug development.
Protein-protein interactions (PPIs) are fundamental to biochemical signaling networks that allow cells to communicate and adapt to an ever-changing environment. On a protein’s surface, the functional groups and their relative orientation ultimately determine how strongly and specifically each protein binds to its partner protein(s) through the formation of many weak non-covalent interactions. Some functional groups contribute disproportionately to PPIs and are thus considered "hot spots."
A central hypothesis in my research is that molecules that reproduce the hot spots of one interacting protein surface in a PPI can competitively inhibit that protein’s interactions with one or more of its partners. To examine this hypothesis, we design, synthesize, and evaluate peptides that adopt well-defined structures that mimic specific protein surfaces.
Specific research areas include 1) divergent synthesis of peptides with multiple, well-defined structures to delineate the effects of sequence and structure on peptide cell permeability, 2) stabilization of peptide polyproline II helices for PPI targeting, and 3) novel solid-phase and hybrid approaches to incorporate non-natural amino acids into peptides.
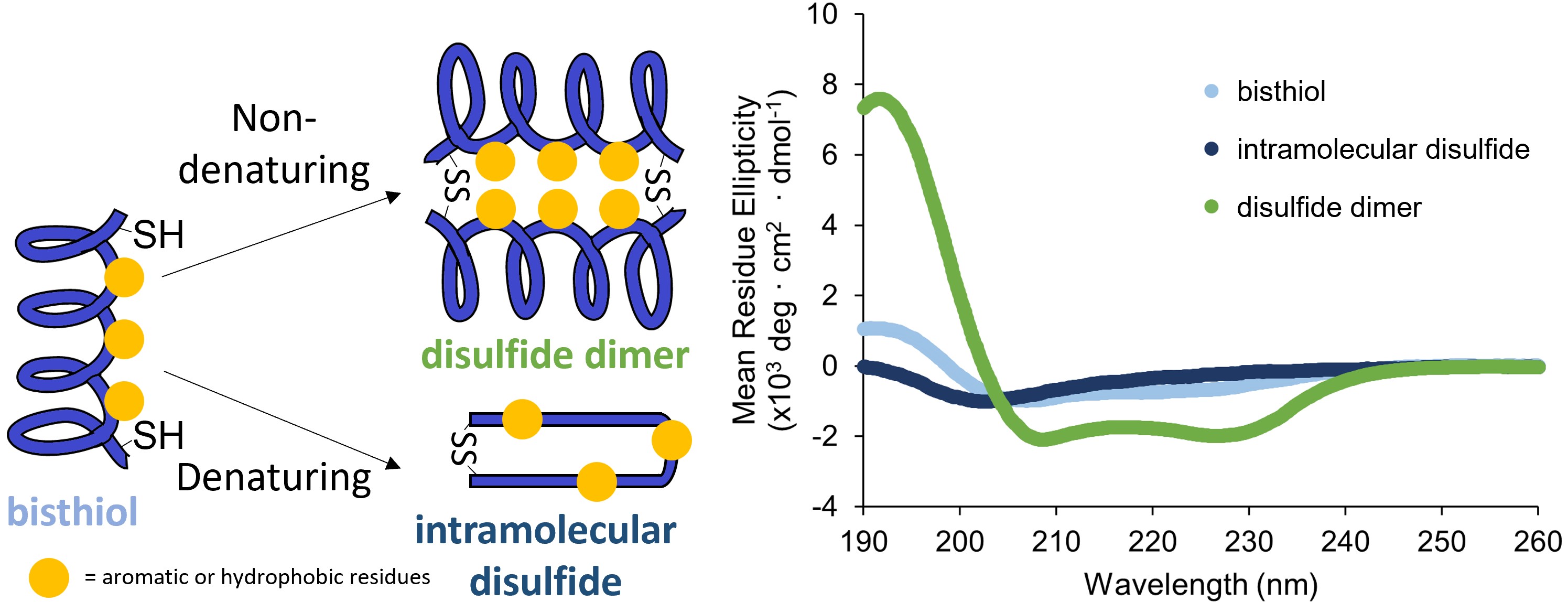
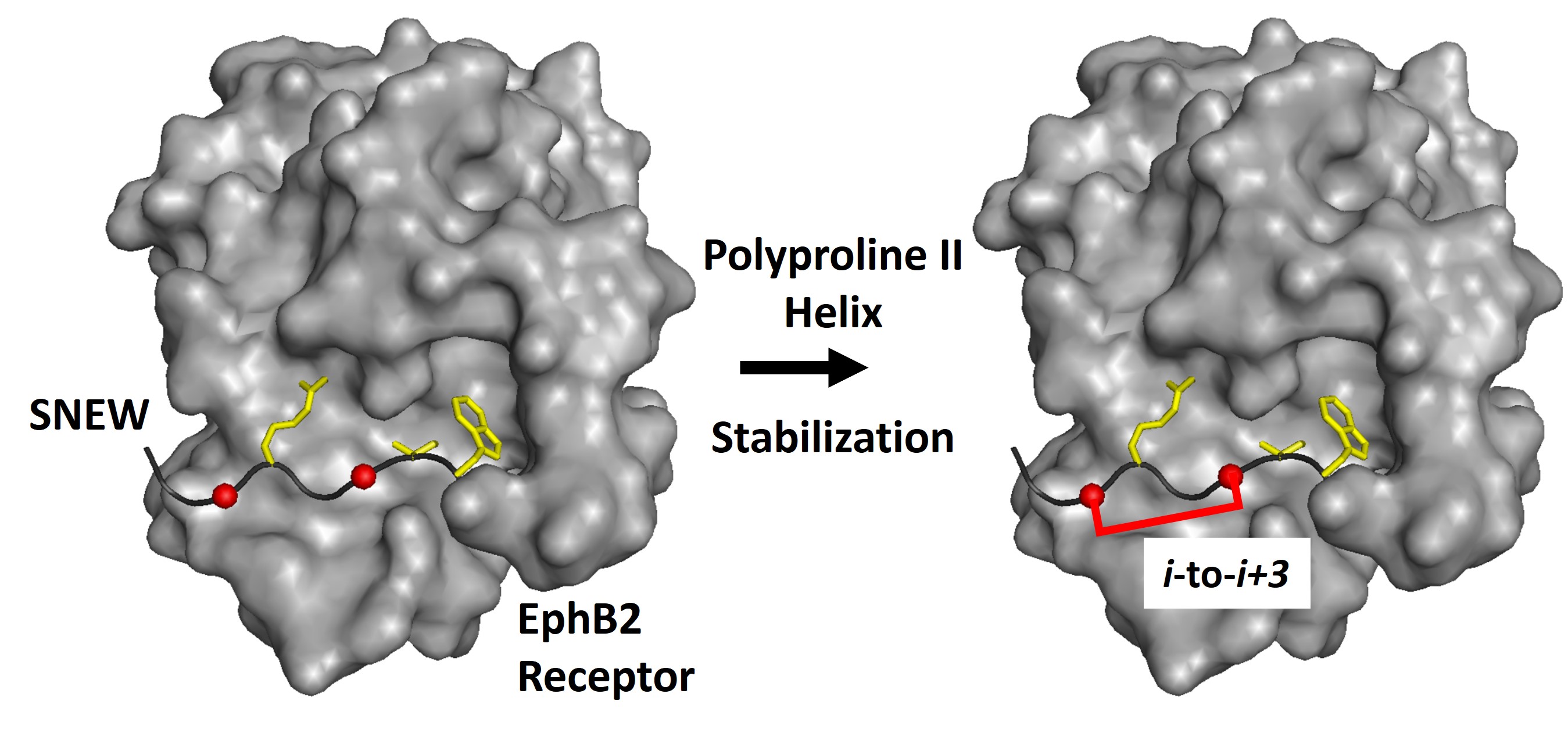
-
Please see Google Scholar for a complete list of publications.
Sawyer, N., Arora, P.S. Hydrogen Bond Surrogate Stabilization of β-Hairpins. ACS Chem. Biol., 2018, 13, 2027.
Sawyer, N., Gassaway, B.M., Haimovich, A.D., Isaacs, F.J., Rinehart, J., Regan, L. Designed phosphoprotein recognition in Escherichia coli. ACS Chem. Biol., 2014, 9, 2502.
Chen, J., Sawyer, N., Regan, L. Protein-protein interactions: general trends in the relationship between binding affinity and interfacial buried surface area. Protein Sci., 2013, 22, 510.