Robert Beer

Associate Professor
Associate Dean for STEM and Pre-Health Education
Inorganic and General Chemistry
Email: [email protected]
Office: JMH 634
Lab: JMH 508
Phone: 718-817-4430
-
- Post-doctoral Research University of Florida
- Ph.D. Massachusetts Institute of Technology
- B.S. University of California at Davis
-
We have studied several types of coordination compounds; large polynuclear metal oxo anions (polyoxometalates) and Schiff base complexes.
We have used single site substitution of the Keggin and Lindquist polytungstates with group V elements to explore both their properties and enhanced reactivity that allows pathways to new compounds and syntheses. One example from our laboratory is the formation and chemistry of [(LNbO)2ZrCp2]6- as shown in the schematic below where the lacunary Keggin ion, L = {α-PW11O39}7-, is represented by a curvilinear shape (J. Am. Chem. Soc., 1999, 121 (38), pp 8953–8954).![One example from our laboratory is the formation and chemistry of [(LNbO)2ZrCp2]6- as shown in the schematic below where the lacunary Keggin ion, L = {α-PW11O39}7-, is represented by a curvilinear shape (J. Am. Chem. Soc., 1999, 121 (38), pp 8953–8954).](/media/review/content-assets/migrated/images/Beer_Research_1.jpg)
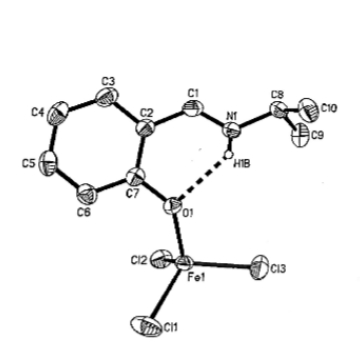
We have also explored the chemistry simple coordination compounds with Schiff base ligands; we have found that neutral salicylaldimine ligands form an intramolecular hydrogen bond, shown below in the iron(III)trichloride adduct of N-isopropylsalicylaldimine (Inorganic Chemistry Communications, 2002, 5, pp. 283–287). This coordination mode is an intermediate in the preparation that leads to the well-known salicylaldimato metal complexes.
-
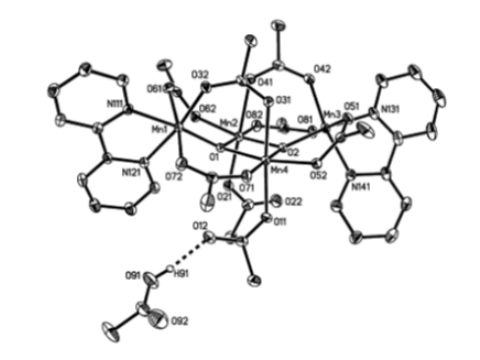
We have found, investigations with fluorinated ligands, that interesting structural and reactivity features arise in models of metalloprotein active sites or chemistry. This is evident in the structure of the tetranuclear manganese complex [Mn4O2(O2CCF3)8(bpy)2] which exhibits a new structural motif – a hydrogen bond to a dangling monodentate acetate ligand (Inorganic Chemistry Communications 2002, 3 pp. 206-210).
We have developed metal complexes as tools to probe the dynamics of macromolecular interactions on fast time scales. In collaboration with colleagues at Einstein College of Medicine fast Fenton footprinting can be achieved between Fe(II)-EDTA/H2O2 and DNA using quench flow methods:
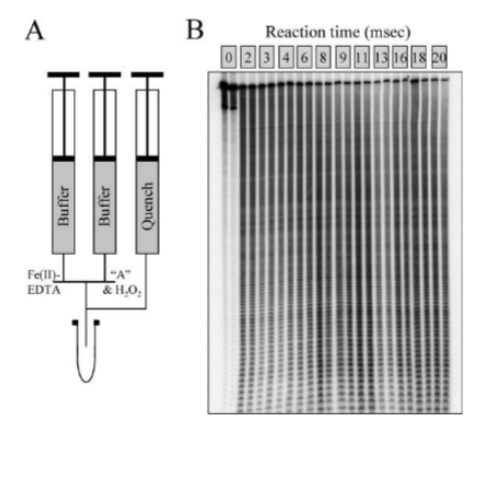
(A) Schematic of quench flow experiment where A is a 282 bp 32 P end-labeled restriction fragment ‘A’ and (B) the dose response autoradiogram of DNA cleavage by OH radical produced. Nucleic Acids Res. 2006, 34(6), e48. -
We have participated in a number of initiatives, programs and grants involving STEM education. Our most recent contribution, is as a Co-PI on a NSF S-STEM grant (awarded 2018-2023). In this grant, we are using multiple high-impact interventions - a research course, summer bridge program, and mentored undergraduate research - to support socioeconomically disadvantaged STEM students at Fordham University. We are studying which interventions work, for which students they work best and under what circumstances they are most effective.
-
Please see Google Scholar for a complete list of publications.