Royall McMahon Ward's Project
Patterns of Flatworm (Bdelloura candida) infestation in the American Horseshoe Crab
The American Horseshoe crab, or Limulus polyphemus, is a marine arthropod that inhabits the Atlantic coastline of North America, from Mexico to Maine (Botton et al. 2017). The limited migration and dispersal are due in part to the short (7-10 days) post-hatch trilobite stage, and the tendency to remain in the intertidal flats and nearshore benthic habitats (Botton et al. 2010). Despite the limited migration of L. polyphemus populations, all throughout L. polyphemus’s distribution range along the Gulf and Atlantic coasts it serves as a host to a marine triclad, Bdelloura candida (Riesgo et al. 2017).
Bdelloura candida is a marine triclad that lives its entire lifecycle on the ventral surfaces of its host, the Limulus polyphemus (Figure 1). B. candida depends on its host for nutrition, where B. candida concentrate on the appendages of the horseshoe crab feeding on the food scraps that the horseshoe crab produces as it eats. B. candida reproduces by depositing stalked cocoons on the gill lamellae (Riesgo et al. 2017).
For our research project, we created a method to document the extent of infestation of adult and juvenile horseshoe crabs. In addition to the quantitative analysis of the extent of infestation, we looked to see if there was a correlation between the size, sex, and condition of the horseshoe crab and the extent of infestation. We were interested to see if a poorer condition horseshoe crabs were more susceptible to being infected, if the size of the host crab affects the prevalence of infestation, or if adult males and female horseshoe crabs are infected at similar frequencies.
We collected our data at Plumb Beach-east, Jamaica Bay, Brooklyn, NY, where horseshoe crab spawning takes place between mid-May to early July. Specimens were randomly selected off the beach to avoid skewing our data.
To document the extent of infestation, we looked at the presence of flatworms in three locations on the horseshoe crab: adult flatworms in the legs, adult flatworms in the book gills, and flatworm egg cocoons in the gill leaflets. Each category was given a score between 0-4, where zero was no presence, and 4 was heavy presence, as a qualitative scale to document the extent of infestation (Figure 4).
The sex of each horseshoe crab was also recorded, using the presence of claspers, leg appendages that male horseshoe crabs use to hold onto the female horseshoe crab during spawning. The prosoma width of the horseshoe crab was also recorded using a metric cloth measuring tape, that was used at the widest part of the prosoma. To record the condition of the horseshoe crab, a previously developed qualitative scale of 1 to 3, with 1 being the best condition, was used (Duffy et al. 2006).
To verify the flatworm species, samples of adult flatworms were extracted and preserved in sea water along with gill leaflets, extracted from freshly deceased horseshoe crabs, and preserved in 70% ethyl alcohol. These samples were photographed using an electron microscope fitted with a camera. A B. candida cocoon is large, has eight developing embryos, and has a short pedicle at its base that attaches itself to the gill (Figure 6) (Shuster et al. 2003). Using these images, we were able to verify that the marine flatworm on the horseshoe crabs of Jamaica Bay were B. candida.
We found a 100% infection rate for adult horseshoe crabs and a 0% infection for juvenile horseshoe crabs, even though they co-occur on the same tidal flats. We found that prosoma width of males show no significant correlation to worm infestation, however when females and males were pooled, there was a significant correlation of adult flatworms on legs (R=0.331, 82 df, p<0.01), adult flatworms on gills (R=0.435, 90 df, p<0.01) and cocoons in gills (R<0.421, 91 df, p<0.01). Female horseshoe crabs had a significantly heavier infestation for all categories than males. We also found that males that were in poorer condition were more heavily infected by adult flatworms in legs (R=0.354, 58 df, p<0.01), and they were more heavily infected by egg cocoons in gills (R=0.435, 58 df, p<0.01). The extent of infestation is extremely significant for horseshoe crabs in Jamaica Bay, where we observed no adult horseshoe crabs that were uninfected.
Images

Figure 1: Bdelloura candida

Figure 4 (left): Heavily infested L. polyphemus, score of 4. |

Figure 4 (right): Uninfected L. polyphemus of adult B. candida in legs, score of O. |
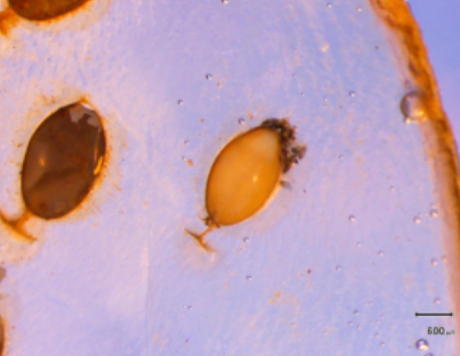
Figure 6: B. candida egg cocoons attached to the inner wall of the gill lamellae. The hatched egg cocoon is a darker brown coloration (left), whereas the lighter tan egg cocoon (right) still have B. candida embryos.
Acknowledgements
I would like to thank Mark Botton, PhD. for his support and guidance, as well as helpful review comments which improved this paper. I would like to thank Mark Hamilton, PhD. for her research scholarship that funded this project. I would also like to thank Fordham University for allowing me the use of their lab and equipment for all lab procedures.
References
Botton, Mark & Colon, Christina & Rowden, John & Elbin, Susan & Kriensky, Debra & Mckown, Kim & Sclafani, Matthew & Madden, Robert. (2017). Effects of a Beach Nourishment Project in Jamaica Bay, New York, on Horseshoe Crab (Limulus polyphemus) Spawning Activity and Egg Deposition. Estuaries and Coasts. 1-14. 10.1007/s12237-017-0337-8.
Botton, Mark & Tankersley, Richard & Loveland, Robert. (2010). Developmental ecology of the American horseshoe crab Limulus polyphemus. Current Zoology. 56.10.1093/czoolo/56.5.550.
Duffy, Erin & Penn, Dustin & Botton, Mark & Brockmann, bullet & Loveland, Robert & Brockmann, H.. (2006). Eye and clasper damage influence male mating tactics in the horseshoe crab, Limulus polyphemus. Journal of Ethology. 24. 67-74. 10.1007/s10164-005-0163-5.
Riesgo, A., Burke, E.A., Laumer, C. et al. Genetic variation and geographic differentiation in the marine triclad Bdelloura candida (Platyhelminthes, Tricladida, Maricola), ectocommensal on the American horseshoe crab Limulus polyphemus . Mar Biol 164, 111 (2017). https://doi.org/10.1007/s00227-017-3132-y
Shuster, C. J., Barlow, R. J., & Brockmann, H. J. (Eds.). (2003). The American Horseshoe Crab.